|
RESEARCH |
General Background
Research in Dr. Gupta's Lab involves developing novel and effective therapies for better management of diseases affecting respiratory system including Lung cancer, Pulmonary arterial hypertension, Fibrosis, Mesothelioma, etc. The Lab has already established multiple delivery strategies by developing novel nanotechnology based delivery systems using biodegradable FDA-approved polymers, lipids and excipients for prolonged circulation and localized accumulation in the lungs. Some of the current work includes repurposing and efficacy enhancement of FDA-approved drug molecules for lung cancer treatment, developing inhalable carriers for pulmonary hypertension, and continuous production of lipid-based inhalable carriers for localized antibiotic delivery in cystic fibrosis associated pulmonary infections. The overall goal is to develop an approach for achieving localized accumulation of the therapy at the disease site, which is simple enough to be scaled up effortlessly for further clinical studies.
We are grateful to the following agencies for funding our research:
ABC, EFG,
We are grateful to the following agencies for funding our research:
ABC, EFG,
Intended Diseases
- Pulmonary Hypertension
- Non-small cell lung cancer
- Mesothelioma
- Cystic Fibrosis associated bacterial infection
- Pulmonary Fibrosis
- Breast Cancer
Recent Publications
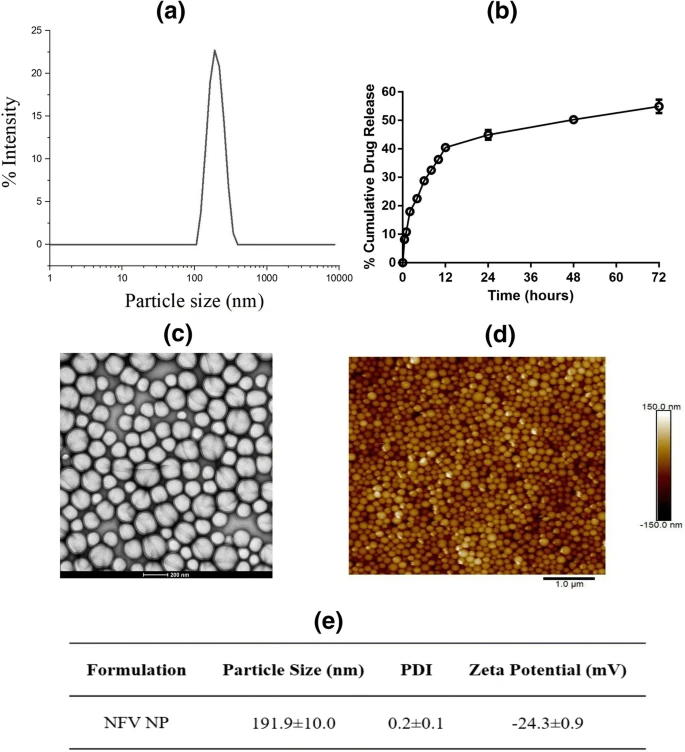
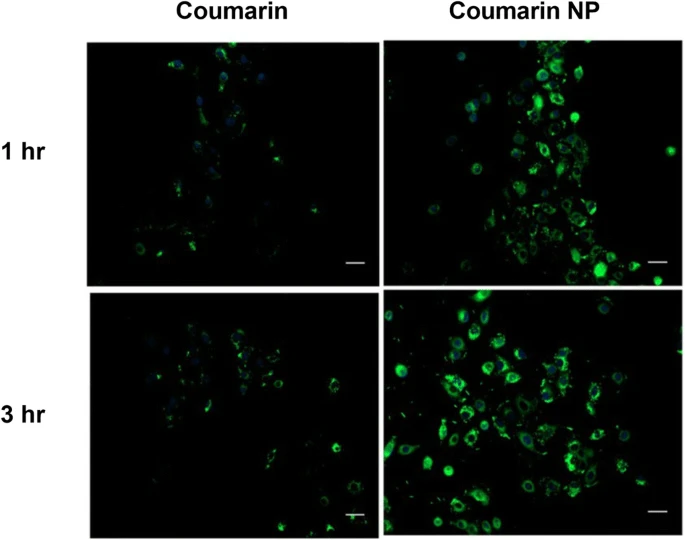
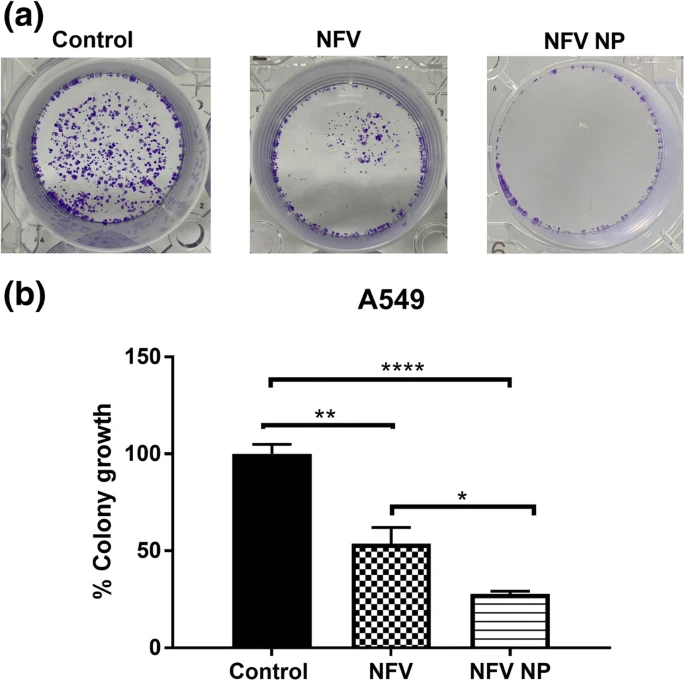
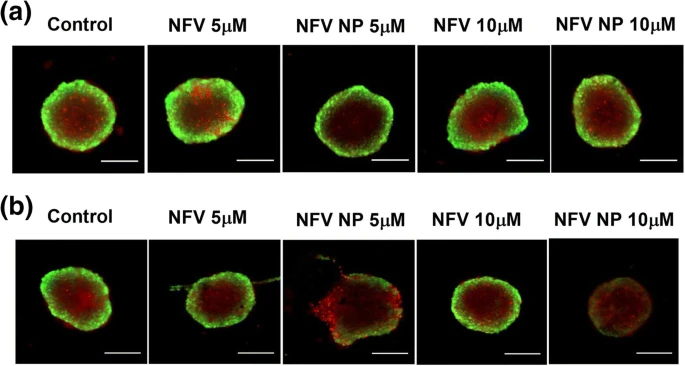
Nelfinavir (NFV), a FDA approved antiretroviral drug, has been reported to exhibit cancer cells growth inhibition and increased apoptosis. However, it requires a higher dose leading to toxicity, thus limiting its potential clinical translation. We aim to develop biodegradable (poly (lactic-co-glycolic acid)) PLGA nanoparticles of nelfinavir and determine their efficacy to treat non-small cell lung cancer (NSCLC). HIV protease inhibitor, NFV, was loaded into PLGA nanoparticles by double emulsion/solvent evaporation method; and nanoparticles were characterized for physicochemical characteristics including morphology and intracellular uptake. Their anti-cancer efficacy in NSCLC was assessed by in vitro assays including cytotoxicity, cellular migration, colony formation; and 3D spheroid culture mimicking in-vivo tumor microenvironment. Studies were also conducted to elucidate effects on molecular pathways including apoptosis, autophagy, and endoplasmic stress. NFV loaded PLGA nanoparticles (NPs) were found to have particle size: 191.1 ± 10.0 nm, zeta potential: −24.3 ± 0.9 mV, % drug loading: 2.5 ± 0.0%; and entrapment efficiency (EE): 30.1 ± 0.5%. NFV NP inhibited proliferation of NSCLC cells compared to NFV and exhibited significant IC50 reduction. From the caspase-dependent apoptosis assays and western blot studies (upregulation of ATF3), it was revealed that NFV NP significantly induced ER stress marker ATF3, cleaved PARP and further caused autophagy inhibition (LC3BII upregulation) leading to increased cellular death. In addition, NFV NP were found to be more efficacious in penetrating solid tumors in ex-vivo studies compared to plain NFV. NFV, a lead HIV protease inhibitor can be repositioned as a NSCLC therapeutic through nanoparticulate delivery. Given its ability to induce apoptosis and efficient tumor penetration capability, NFV loaded PLGA nanoparticulate systems provide a promising delivery system in NSCLC treatment.
New drug and dosage form development faces significant challenges, especially in oncology, due to longer development cycle and associated scale -up complexities. Repurposing of existing drugs with potential anti -cancer activity into new therapeutic regimens provides a feasible alternative. In this project, amodiaquine (AQ), an anti -malarial drug, has been explored for its anti -cancer efficacy through formulating inhalable nanoparticulate systems using high -pressure homogenization (HPH) with scale -up feasibility and high reproducibility. A 3 2 multifactorial design was employed to better understand critical processes (probe homogenization speed while formulating coarse emulsion) and formulation parameters (concentration of cationic polymer in external aqueous phase) so as to ensure product quality with improved anticancer efficacy in non -small cell lung cancer (NSCLC). Optimized AQ loaded nanoparticles (AQ NP) were evaluated for physicochemical properties, stability profile, in -vitro aerosol deposition behavior, cytotoxic potential against NSCLC cells in -vitro and in 3D simulated tumor spheroid model. The highest probe homogenization speed (25 ,000rpm) resulted in lower particle size. Incorporation of cationic polymer, polyethyleneimine (0.5% w/v) resulted in high drug loading efficiencies at optimal drug quantity of 5mg. Formulated nanoparticles (liquid state) exhibited an aerodynamic diameter of 4. 7±0.1µm and fine particle fraction of 81.0±9. 1 %, indicating drug deposition in the respirable airways. Cytotoxicity studies in different NSCLC cell lines revealed significant reduction in IC50 values with AQ -loaded nanoparticles compared to plain drug, along with significant cell migration inhibition (scratch assay) and reduced % colony growth (clonogenic assay) in A549 cells with AQ NP. Moreover, 3D simulated spheroid studies revealed efficacy of nanoparticles in penetration to tumor core, and growth inhibition. AQ’s autophagy inhibition ability significantly increased (increased LC3B -II levels) with nanoparticle encapsulation, along with moderate improvement in apoptosis induction (Caspase -3 levels). No impact was observed on HUVEC angiogenesis suggesting alternative anticancer mechanisms. To conclude, amodiaquine can be a promising candidate for repurposing to treat NSCLC while delivering inhalable nanoparticles developed using a scalable HPH process. Despite the involvement of complex parameters, application of DoE has simplified the process of product and process optimization.

The purpose of this study was to design and evaluate chitosan dispersed lipid vesicles (chitosomes) as potential delivery carriers for repurposing metformin (Met) against malignant pleural mesothelioma. Chitosomes were prepared by directly hydrating the thin lipid film using chitosan solution as hydration medium, instead of using it as a coating agent. Developed chitosomes demonstrated spherical morphology, positive surface charge (~30 mV) and ~60% encapsulation efficiency. The calorimetric studies and X-ray diffraction pattern of Met-loaded chitosomes confirmed the successful encapsulation of Met inside the chitosome vesicles. Optimized chitosome formulation showed ~70% drug release in 72 h, displaying prolonged and controlled release of drug. Results demonstrated that Met encapsulated chitosomes possessed enhanced cellular internalization and improved cytotoxic potential. Our findings also supported inhibitory activity of chitosomes against metastatic property of pleural mesothelioma cells. The in-vitro tumor simulation studies further established anti-tumor activity of Met encapsulated chitosomes as supported by reduction in tumor volume and presence of minimal viable cells in tumor mass. The obtained results establish the effectiveness of chitosomes as delivery carrier for Met as treatment alternative for malignant pleural mesothelioma.

This exploration is aimed at developing sorafenib (SF)-loaded cationically-modified polymeric nanoparticles (NPs) as inhalable carriers for improving the therapeutic efficacy of SF against non-small cell lung cancer (NSCLC). The NPs were prepared using a solvent evaporation technique while incorporating cationic agents. The optimized NPs were characterized by various physicochemical parameters and evaluated for their aerosolization properties. Several in-vitro evaluation studies were performed to determine the efficacy of our delivery carriers against NSCLC cells. Optimized nanoparticles exhibited an entrapment efficiency of ~40%, 80%). In-vitro evaluation also resulted in a superior ability to inhibit cancer metastasis. 3D-tumor simulation studies further established the anti-cancer efficacy of NPs as compared to just SF. The localized delivery of SF-loaded nanoparticles resulted in improved anti-tumor activity as compared to SF alone. Therefore, this strategy displays great potential as a novel treatment approach against certain lung cancers.
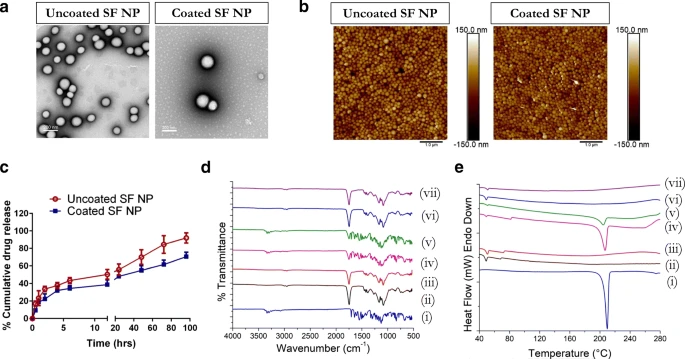
Drug repurposing is on the rise as an atypical strategy for discovery of new molecules, involving use of pre-existing molecules for a different therapeutic application than the approved indication. Using this strategy, the current study aims to leverage effects of quinacrine (QA), a well-known anti-malarial drug, for treatment of non-small cell lung cancer (NSCLC). For respiratory diseases, designing a QA loaded inhalable delivery system has multiple advantages over invasive delivery. QA-loaded nanoparticles (NPs) were thus prepared using polyethyleneimine (PEI) as a cationic stabilizer. While the use of PEI provided cationic charge on the particles, it also mediated a burst release of QA and demonstrated potential particle toxicity. These concerns were circumvented by coating nanoparticles with bovine serum albumin (BSA), which retained the cationic charge, reduced NP toxicity and modulated QA release. Prepared nanoparticles were characterized for physicochemical properties along with their aerosolization potential. Therapeutic efficacy of the formulations was tested in different NSCLC cells. Mechanism of higher anti-proliferation was evaluated by studying cell cycle profile, apoptosis and molecular markers involved in the progression of lung cancer. BSA coated QA nanoparticles demonstrated good aerosolization potential with a mass median aerodynamic diameter of significantly less than 5 µm. Nanoparticles also demonstrated improved therapeutic efficacy against NSCLC cells in terms of low IC50 values, cell cycle arrest at G2/M phase and autophagy inhibition leading to increased apoptosis. BSA coated QA NPs also demonstrated enhanced therapeutic efficacy in a 3D cell culture model. The present study thus lays solid groundwork for pre-clinical and eventual clinical studies as a standalone therapy and in combination with existing chemotherapeutics.

This study was aimed at developing a nanoparticle strategy to overcome acquired resistance against erlotinib in non-small cell lung cancer (NSCLC). To load erlotinib on biodegradable PLGA nanoparticles, erlotinib-cyclodextrin (Erlo-CD) complex was prepared using β-cyclodextrin sulfobutyl ether, which was in turn loaded in the core of PLGA nanoparticles using multiple emulsion solvent evaporation. Nanoparticles were characterized for size distribution, entrapment and loading efficiency, in-vitro release, and therapeutic efficacy against different lung cancer cells. Effect of formulation on cell cycle, apoptosis, and other markers was evaluated using flow cytometry and western blotting studies. The efficacy of optimized nanoformulation was evaluated using a clinically relevant in-vitro 3D-spheroid model. Results showed that Erlo-CD loaded nanoparticles (210 ± 8 nm in size) demonstrated 3-fold higher entrapment (61.5 ± 3.2% vs 21.9 ± 3.7% of plain erlotinib loaded nanoparticles) with ~5% loading efficiency and sustained release characteristics. Developed nanoparticles demonstrated significantly improved therapeutic efficacy against NSCLC cells in terms of low IC50 values and suppressed colony forming ability of cancer cells, increased apoptosis, and autophagy inhibition. Interestingly, 3D spheroid study demonstrated better anticancer activity of Erlo-CD nanoparticles compared to plain erlotinib. Present study has shown a premise to improve therapeutic efficacy against erlotinib-resistant lung cancer using modified nanoErlo formulations.

Exploratory Ideas
- Is dry powder inhalation being beneficial compared to nebulized liquid formulations in treating lung cancer?
- Investigation on solubility enhancement of poorly soluble drugs through high-pressure homogenization.
- Is USP dissolution apparatus physiologically relevant or limited to quality control?
- Can the pharmaceutical industry completely rely on continuous manufacturing process with no intermediate quality control and checks?




